Apr 15, 2022The equation for the hydrolysis of glyceryl triethanoate is 3C2H4O2 + C3H5O3 → 3C2H5OH + C3H5 (OH)3. This reaction involves the formation of ethanol and glycerol. Explanation: The equation for the hydrolysis of glyceryl triethanoate, also known as triacetyl glycerol, can be represented as: 3C2H4O2 + C3H5O3 → 3C2H5OH + C3H5 (OH)3
OneClass: What is the equation for the hydrolysis of glyceryl triethanoate
Is the esterification of glyceryl ethanoate a reversible equation? Organic Chemistry Welcome to Organic Chemistry Definition of ‘Chemistry’ and ‘Organic’ 1 Answer

Source Image: mdpi.com
Download Image
Section: Chapter Questions Problem 25.9EP: What are the products of the complete hydrolysis of a triacylglycerol? See similar textbooks Related questions Concept explainers Question equation for the hydrolysis of glyceryl triethanoate Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps with 1 images

Source Image: homework.study.com
Download Image
Kinetics Study of the Solid-State Acid Hydrolysis of Chitosan: Evolution of the Crystallinity and Macromolecular Structure | Biomacromolecules
1,692 solutions Modern Chemistry 1st Edition • ISBN: 9780547586632 (2 more) Jerry L. Sarquis, Mickey Sarquis 2,184 solutions More related questions chemistry Acid-catalyzed transesterification and Fischer esterification take place by nearly identical mechanisms.

Source Image: homework.study.com
Download Image
Equation For The Hydrolysis Of Glyceryl Triethanoate
1,692 solutions Modern Chemistry 1st Edition • ISBN: 9780547586632 (2 more) Jerry L. Sarquis, Mickey Sarquis 2,184 solutions More related questions chemistry Acid-catalyzed transesterification and Fischer esterification take place by nearly identical mechanisms.
The hydrolysis is mediated by an enzyme, glyceryl triethanolamine amidohydrolase. The condensed structural formulas for the reactants and products are shown below. Step 2/2 The hydrolysis of glyceryl triethanoate can be diagramed using the following equation.
What is the mechanism for the hydrolysis of glyceryl triethanoate? | Homework.Study.com
Answer and Explanation: 1 Glyceryl triethanoate, also called triacetin, is a triglyceride. Its structure is shown as follows. Upon hydrolysis through reaction with a strong base such as
Write an equation for the acid hydrolysis of glyceryl trilaurate (trilaurin). | Homework.Study.com

Source Image: homework.study.com
Download Image
Solved] Answer the following questions, and for the drawing questions… | Course Hero
Answer and Explanation: 1 Glyceryl triethanoate, also called triacetin, is a triglyceride. Its structure is shown as follows. Upon hydrolysis through reaction with a strong base such as
Source Image: coursehero.com
Download Image
OneClass: What is the equation for the hydrolysis of glyceryl triethanoate
Apr 15, 2022The equation for the hydrolysis of glyceryl triethanoate is 3C2H4O2 + C3H5O3 → 3C2H5OH + C3H5 (OH)3. This reaction involves the formation of ethanol and glycerol. Explanation: The equation for the hydrolysis of glyceryl triethanoate, also known as triacetyl glycerol, can be represented as: 3C2H4O2 + C3H5O3 → 3C2H5OH + C3H5 (OH)3

Source Image: oneclass.com
Download Image
Kinetics Study of the Solid-State Acid Hydrolysis of Chitosan: Evolution of the Crystallinity and Macromolecular Structure | Biomacromolecules
Section: Chapter Questions Problem 25.9EP: What are the products of the complete hydrolysis of a triacylglycerol? See similar textbooks Related questions Concept explainers Question equation for the hydrolysis of glyceryl triethanoate Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps with 1 images

Source Image: pubs.acs.org
Download Image
Solved 3. Equation for the hydrolysis of glyceryl | Chegg.com
May 6, 2023CH2 (OOCCH3)C (OOCH3)CH (OOCCH3)2 + 3H2O → C3H5 (OH)3 + 3CH3COOH In this equation, C3H5 (OH)3 represents glycerol, while CH3COOH represents acetic acid. The hydrolysis of triacetin is an example of a saponification reaction, which is commonly used in the production of soap. To learn more about compound, click here: brainly.com/question/13516179
Source Image: chegg.com
Download Image
⏩SOLVED:Write the equation for the acid hydrolysis of glyceryl… | Numerade
1,692 solutions Modern Chemistry 1st Edition • ISBN: 9780547586632 (2 more) Jerry L. Sarquis, Mickey Sarquis 2,184 solutions More related questions chemistry Acid-catalyzed transesterification and Fischer esterification take place by nearly identical mechanisms.
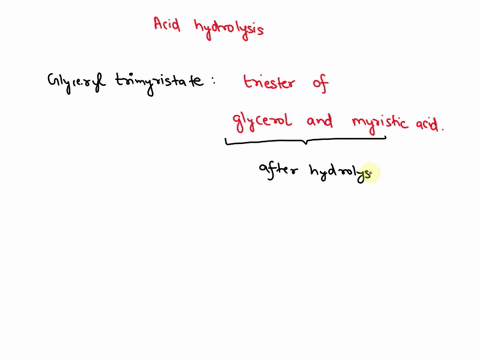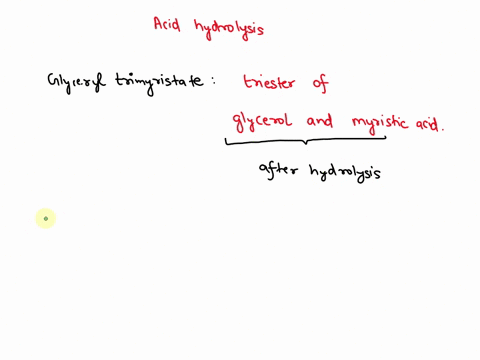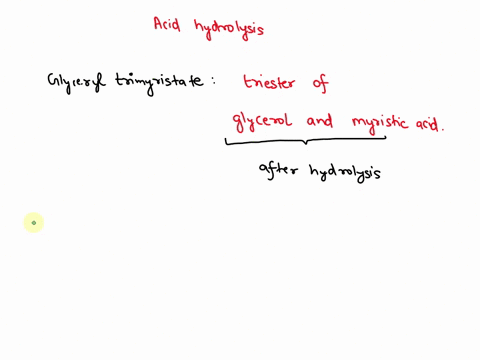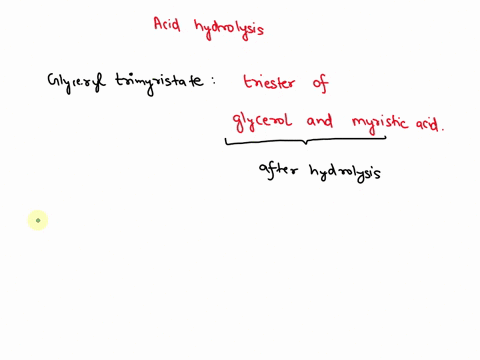
Source Image: numerade.com
Download Image
Chapter 15 Vocab Flashcards | Quizlet
The hydrolysis is mediated by an enzyme, glyceryl triethanolamine amidohydrolase. The condensed structural formulas for the reactants and products are shown below. Step 2/2 The hydrolysis of glyceryl triethanoate can be diagramed using the following equation.

Source Image: quizlet.com
Download Image
Solved] Answer the following questions, and for the drawing questions… | Course Hero
Chapter 15 Vocab Flashcards | Quizlet
Is the esterification of glyceryl ethanoate a reversible equation? Organic Chemistry Welcome to Organic Chemistry Definition of ‘Chemistry’ and ‘Organic’ 1 Answer
Kinetics Study of the Solid-State Acid Hydrolysis of Chitosan: Evolution of the Crystallinity and Macromolecular Structure | Biomacromolecules ⏩SOLVED:Write the equation for the acid hydrolysis of glyceryl… | Numerade
May 6, 2023CH2 (OOCCH3)C (OOCH3)CH (OOCCH3)2 + 3H2O → C3H5 (OH)3 + 3CH3COOH In this equation, C3H5 (OH)3 represents glycerol, while CH3COOH represents acetic acid. The hydrolysis of triacetin is an example of a saponification reaction, which is commonly used in the production of soap. To learn more about compound, click here: brainly.com/question/13516179