Find the empirical formula and the molecular formula of an organic compound from the data given below: C = 7 5 . 9 2 % , H = 6 . 3 2 % and N = 1 7 . 7 6 % The vapour density of the compound is 3 9 . 5 .
Enantio- and Diastereoselective Michael Reaction of 1,3-Dicarbonyl Compounds to Nitroolefins Catalyzed by a Bifunctional Thiourea | Journal of the American Chemical Society
Chemistry Percentage Composition Question Molecular formula of a compound is C 6 H 18 O 3. Find its empirical formula. Solution Verified by Toppr Molecular formula = C 6 H 18 O 3 Take the common multiple Molecular formula = ( C 2 H 6 O) 3 Molecular formula = ( E m p i r i c a l f o r m u l a) n Thus, empirical formula = C 2 H 6 O

Source Image: m.youtube.com
Download Image
The answers are 5C, 1N, and 5H. The empirical formula is C 5 H 5 N, which has a molar mass of 79.10 g/mol. To find the actual molecular formula, divide 240, the molar mass of the compound, by 79.10 to obtain 3. So the formula is three times the empirical formula, or C 15 H 15 N 3.

Source Image: youtube.com
Download Image
Percent Composition, Empirical and Molecular Formulas – Presentation Chemistry In a molecular formula, it states the total number of atoms of each element in a molecule. For example, the molecular formula of glucose is C6H 12O6, and we do not simplify it into CH 2O. And for each compound, they all have a molecular formula, but some can be similar, and those are called isomers, which are common in organic chemistry.

Source Image: patents.google.com
Download Image
What Is The Empirical Formula Of C6h18o3
In a molecular formula, it states the total number of atoms of each element in a molecule. For example, the molecular formula of glucose is C6H 12O6, and we do not simplify it into CH 2O. And for each compound, they all have a molecular formula, but some can be similar, and those are called isomers, which are common in organic chemistry. There are 4 easy steps to find the molar mass of C6H18O3 based on its chemical formula. 1. Count The Number of Each Atom The first step to finding the molar mass is to count the number of each atom present in a single molecule using the chemical formula, C6H18O3: 2. Find Atomic Mass of Each Element
EP0410176A1 – 2-Aminopentanoic acid compounds and their use as immunosuppressants – Google Patents
Jul 21, 2022Determining Empirical Formulas. An empirical formula tells us the relative ratios of different atoms in a compound. The ratios hold true on the molar level as well. Thus, H 2 O is composed of two atoms of hydrogen and 1 atom of oxygen. Likewise, 1.0 mole of H 2 O is composed of 2.0 moles of hydrogen and 1.0 mole of oxygen.We can also work backwards from molar ratios because if we know the Fórmula empírica y molecular – El Gen Curioso

Source Image: elgencurioso.com
Download Image
Chapter 6 Chemical Composition. – ppt video online download Jul 21, 2022Determining Empirical Formulas. An empirical formula tells us the relative ratios of different atoms in a compound. The ratios hold true on the molar level as well. Thus, H 2 O is composed of two atoms of hydrogen and 1 atom of oxygen. Likewise, 1.0 mole of H 2 O is composed of 2.0 moles of hydrogen and 1.0 mole of oxygen.We can also work backwards from molar ratios because if we know the
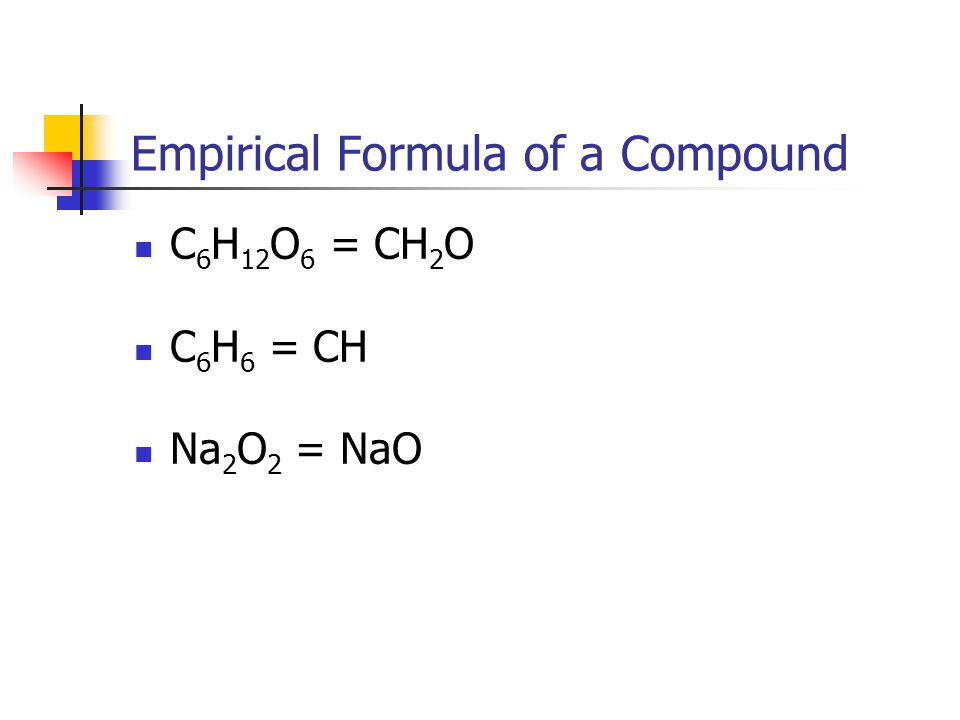
Source Image: slideplayer.com
Download Image
Enantio- and Diastereoselective Michael Reaction of 1,3-Dicarbonyl Compounds to Nitroolefins Catalyzed by a Bifunctional Thiourea | Journal of the American Chemical Society Find the empirical formula and the molecular formula of an organic compound from the data given below: C = 7 5 . 9 2 % , H = 6 . 3 2 % and N = 1 7 . 7 6 % The vapour density of the compound is 3 9 . 5 .

Source Image: pubs.acs.org
Download Image
Percent Composition, Empirical and Molecular Formulas – Presentation Chemistry The answers are 5C, 1N, and 5H. The empirical formula is C 5 H 5 N, which has a molar mass of 79.10 g/mol. To find the actual molecular formula, divide 240, the molar mass of the compound, by 79.10 to obtain 3. So the formula is three times the empirical formula, or C 15 H 15 N 3.
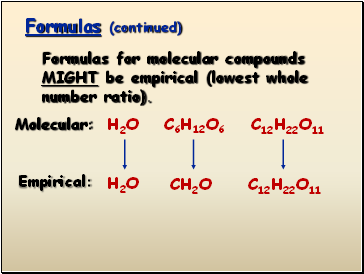
Source Image: sliderbase.com
Download Image
From percentage to formula – ppt video online download Molecular Formulas: The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule.Both Benzene (C 6 H 6, molar mass = 78.12g/mol) and acetylene (C 2 H 2, molar mass = 26.04g/mol) have the same percent composition (92.24 mass% carbon and 7.76% hydrogen) and the empirical formula, CH.

Source Image: slideplayer.com
Download Image
Furaneol Formula – C6H8O3 – Over 100 million chemical compounds | CCDDS In a molecular formula, it states the total number of atoms of each element in a molecule. For example, the molecular formula of glucose is C6H 12O6, and we do not simplify it into CH 2O. And for each compound, they all have a molecular formula, but some can be similar, and those are called isomers, which are common in organic chemistry.

Source Image: molinstincts.com
Download Image
Empirical Formula & Molecular Formula Determination From Percent Composition – YouTube There are 4 easy steps to find the molar mass of C6H18O3 based on its chemical formula. 1. Count The Number of Each Atom The first step to finding the molar mass is to count the number of each atom present in a single molecule using the chemical formula, C6H18O3: 2. Find Atomic Mass of Each Element

Source Image: youtube.com
Download Image
Chapter 6 Chemical Composition. – ppt video online download
Empirical Formula & Molecular Formula Determination From Percent Composition – YouTube Chemistry Percentage Composition Question Molecular formula of a compound is C 6 H 18 O 3. Find its empirical formula. Solution Verified by Toppr Molecular formula = C 6 H 18 O 3 Take the common multiple Molecular formula = ( C 2 H 6 O) 3 Molecular formula = ( E m p i r i c a l f o r m u l a) n Thus, empirical formula = C 2 H 6 O
Percent Composition, Empirical and Molecular Formulas – Presentation Chemistry Furaneol Formula – C6H8O3 – Over 100 million chemical compounds | CCDDS Molecular Formulas: The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule.Both Benzene (C 6 H 6, molar mass = 78.12g/mol) and acetylene (C 2 H 2, molar mass = 26.04g/mol) have the same percent composition (92.24 mass% carbon and 7.76% hydrogen) and the empirical formula, CH.